5+ orbital diagram for neon
Ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. What is an orbital diagram.

Electron Configurations Of Ions Video Khan Academy
Fill in the orbital energy diagram for the neon atom.

. Write electron configuration of neon. In the iodine ground-state electron configuration five. The electron configuration of neon is.
In the case of Neon we have the electron configuration is He 2s2 2p6 for this particular element. Reduced electronic configuration Ne. Orbital diagrams Orbital box diagrams of all elements are mentioned in the.
The electron configuration of an atom is 1s 2 2s 2 2p 6. 000084 gcm 3. Below is the electronic diagram.
What is the orbital diagram for iodine. The Basics of Orbital Diagrams. There are different types of orbitals that all have different energy levels.
That is the number of electrons and protons in the sodium atom is eleven. Find electrons of neon. These orbitals are filled with electrons the amount of electrons depends on which.
This is the final precise form for the electron configuration of the element. So the electron configuration of. 1s 2s.
Heres how you can draw the orbital diagram of neon step by step. Electronic configuration of the Neon atom. He 2s 2 2p 6.
In writing the electron configuration for neon the first two electrons will go in. Neon is the tenth element with a total of 10 electrons. 1s 2 2s 2 2p 6.
The orbital diagram will be filled in the same order as described by the Aufbau principle. The full orbital diagram for neon is shown. Molecular Orbital Diagram of Neon Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC IIT JEE CBSE NEETWat.
The atomic number of an element is the number of electrons and protons in that element. The number of electrons in the atom is. Chemistry questions and answers.
Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. An orbital diagram is similar to electron configuration except that instead of indicating the atoms. The order in which the orbitals are filled with electrons from lower energy to higher energy is.
2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining. The above orbital diagram shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons and the 2p subshell has 6 electrons.

The Speciation Of Americium Cations In Neat Water Implicated From Dft Studies Inorganic Chemistry
![]()
Neon Orbital Diagram Electron Configuration And Valence Electrons

Ne Neon Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

Electron Configuration Of Technetium Tc Lesson Youtube

How Many D Electrons Are Present In The Ground State Electron Configuration Of Cu Quora

Phosphorus Electron Configuration Youtube
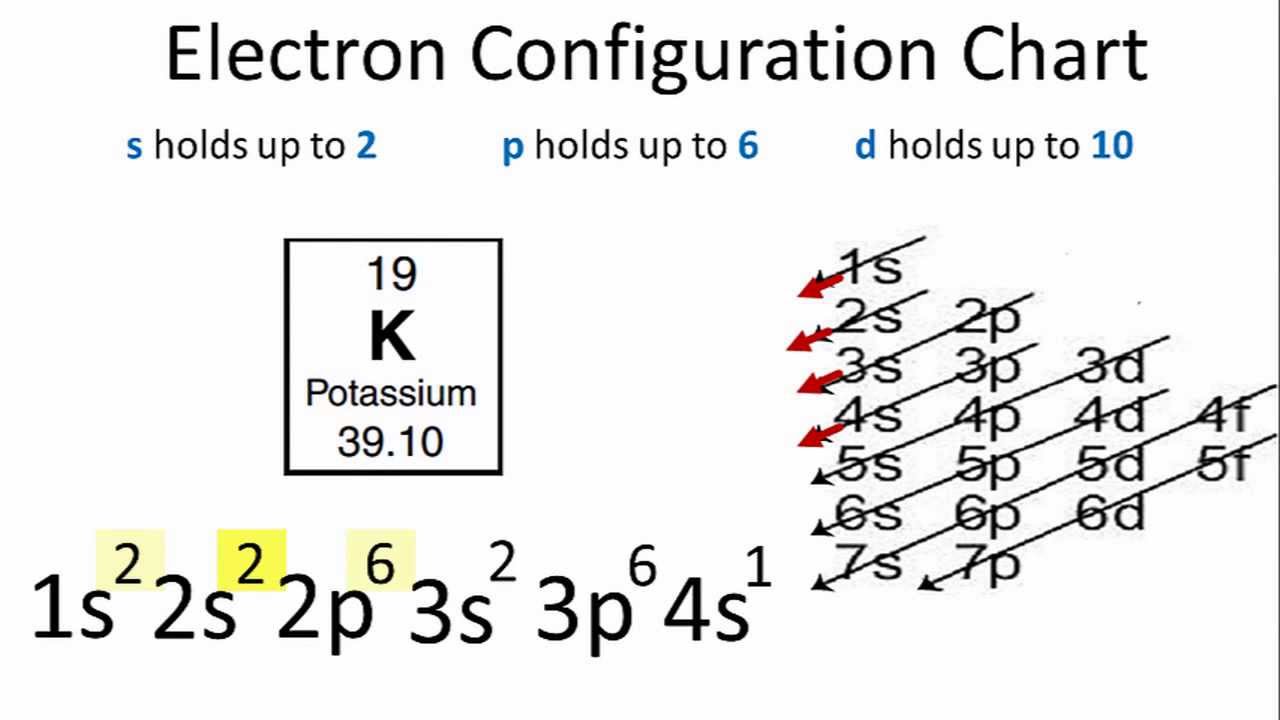
Electron Configuration For Potassium K

Create An Orbital Diagram For Nitrogen Neon Ppt Download
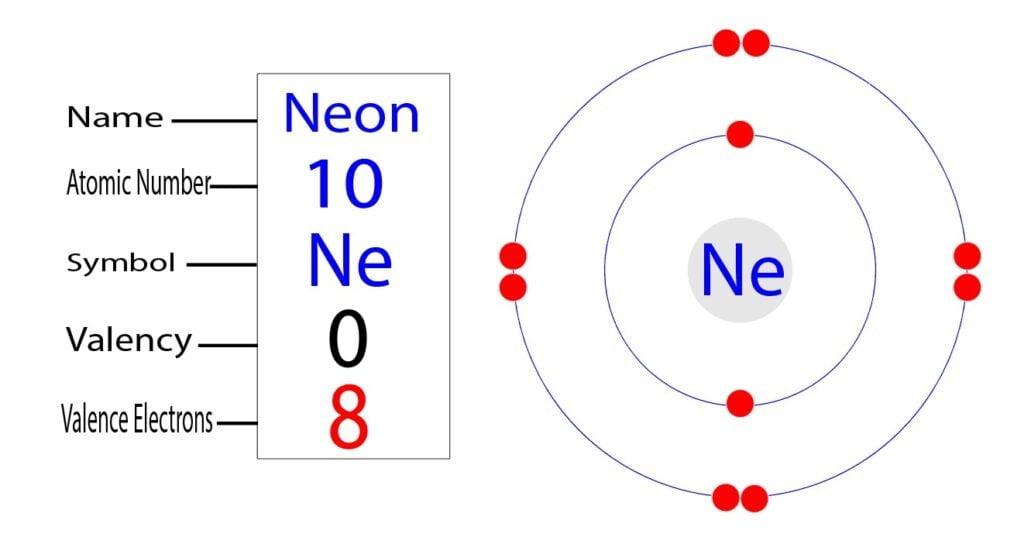
Neon Ne Electron Configuration And Orbital Diagram
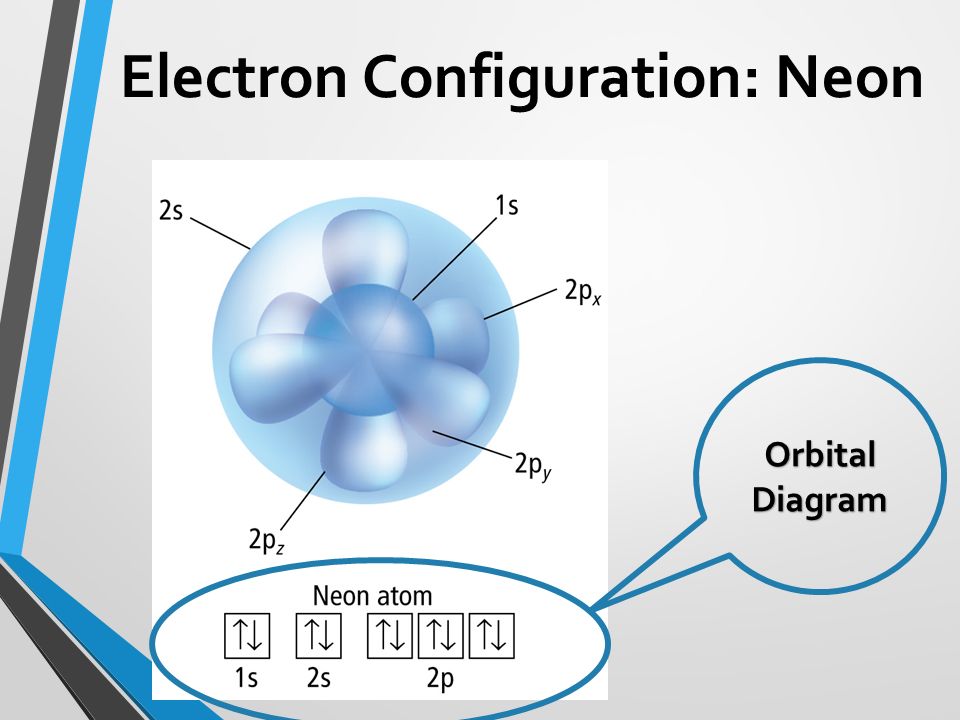
Electrons In Atoms Chapter 5 Chapter Big Idea The Atoms Of Each Element Have A Unique Arrangement Of Electrons Ppt Download
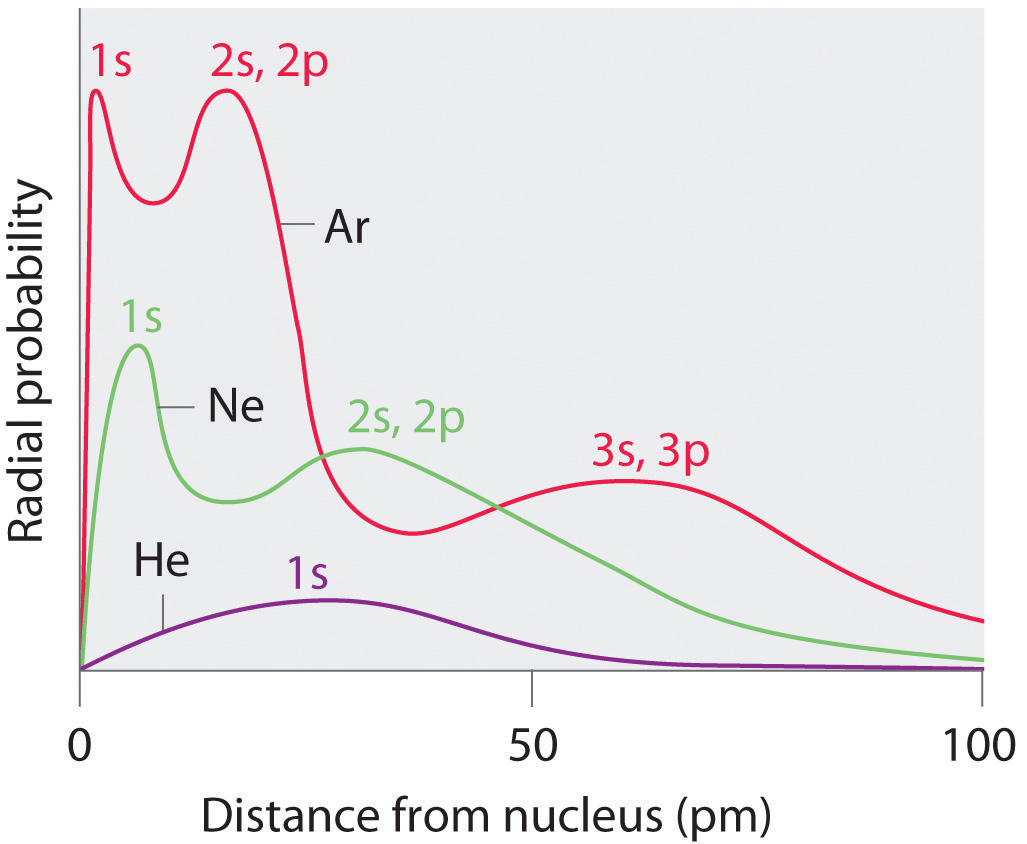
Sizes Of Atoms And Ions

Create An Orbital Diagram For Nitrogen Neon Ppt Download
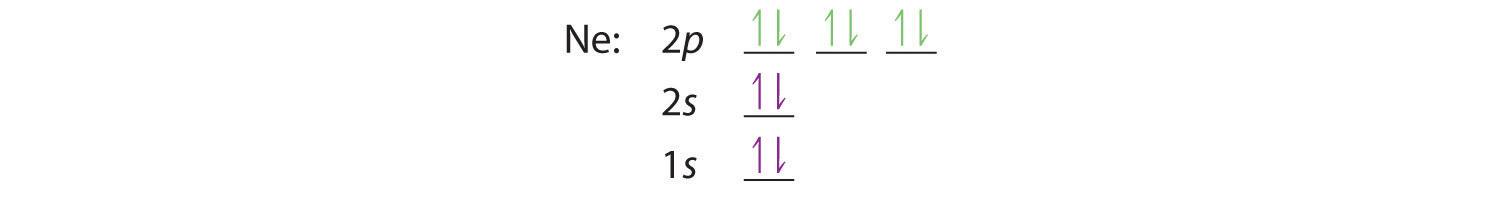
Building Up The Periodic Table

How To Write The Orbital Diagram For Neon Ne Youtube
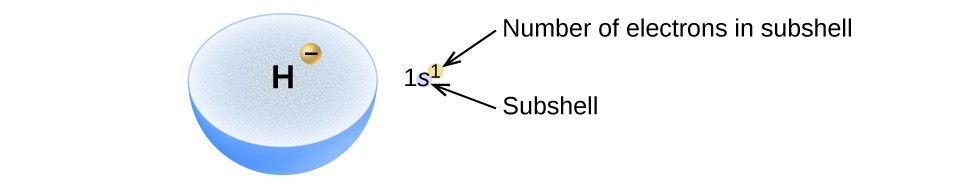
Electronic Structure Of Atoms Electron Configurations General Chemistry I Course Hero
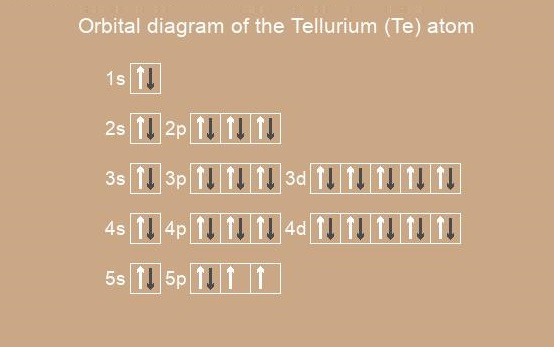
Tellurium Electron Configuration Atomic Number Mass Uses

Neon Orbital Diagram Electron Configuration And Valence Electrons